Achieving Repeatability in Staining with a Modified NEI Scale
By: Dr. Kenneth Sall

Corneal fluorescein staining, which assesses the rate and process of healing, is one of the most important components of dry eye studies.
Yet despite providing such a crucial trial endpoint, staining is difficult to measure consistently – we’re still figuring out the best way to guarantee reproducibility across PIs, visits, and sites.
Over the years, this challenge has prompted a transformation in how we measure and grade corneal fluorescence staining.
The Evolution of Grading Scales for Staining
Today, clinical trials typically use the Oxford scale, the OSS scale, or the NEI scale. The NEI scale — the most common of the three – has evolved significantly, bringing us closer to more reproducible staining.
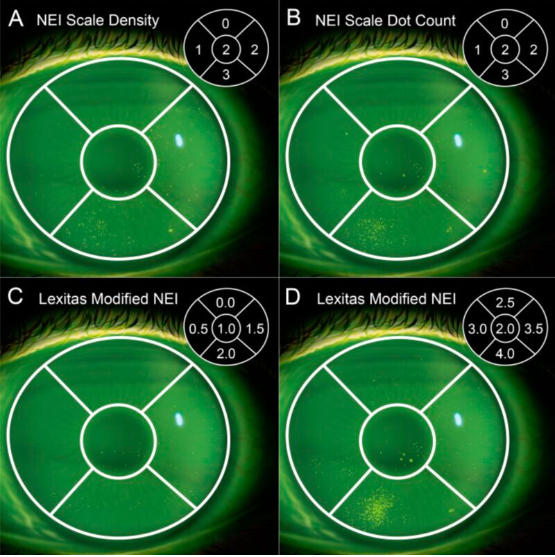
Originally, the NEI scale measured density on a 4.0 scale, with three sections. However, because density is specific and somewhat subjective, we struggled to train PIs and maintain consistency across trials. So, we updated this scale to measure the total number of dots instead of density of dots. Right away, PIs much preferred counting dots to assessing density, and the NEI scale quickly rose in popularity.
But despite the widespread adoption of this simple, more streamlined scale, we still struggled to reliably show efficacy in staining, and many drugs continued to fail. The dot-counting method, thought easy for graders, wasn’t detailed enough to detect small – yet clinically significant – changes.
To rectify this, companies began modifying the NEI scale to improve precision. One such approach, Lexitas’ modified scale, uses a nine-point system, instead of four, with half-points to capture more granular data.
The new nine-point scale showed promise. But how did it stack up against other methods?
Evaluating Scale Success: Designing Trials for Reproducibility
Together with a group of scientists, I designed and ran trials to test and validate the Lexitas scale. In addition to testing the scale itself, we made sure to minimize extraneous variables – from the type of computers to the profile of each grader – that might compromise the study.
For the assessment, we used both photographs and illustrations of the cornea to avoid issues with blurry or unfocused photos. After overlaying the NEI grid on top of the images, our expert graders examined and analyzed the cornea. We chose graders with multiple years of experience in trial settings to make sure that skill level didn’t affect the tests.
In addition, each grader used the same monitors, mouse, screens, software, etc. This helped us ensure repeatability between graders (inter-exam repeatability). To maintain repeatability within a single grader (intra-exam repeatability), we invited each grader back to complete a second assessment, and compared their initial and final results.
Achieving Repeatability with the Lexitas Scale
After our evaluation concluded, we found that the Lexitas scale performed equal to or better than all other scales, and demonstrated both inter-exam and intra-exam repeatability.
Initially, we wondered whether graders would perform worse on Lexitas’ more complex 9-point scale but found that the number of points didn’t matter. Our graders quickly and easily learned the Lexitas scale during training. They also made use of the accompanying reference materials throughout the study.
While it’s best practice to use reference materials during any evaluation, the detailed 9-point scale – compared with the more familiar 4-point scale – helped encourage this behavior and maintain grading consistency. With clear instructions, effective training, and thorough reference material, PIs can quickly learn and adopt these more detailed, reproducible methods.
Going Beyond the Clinic – Using the Lexitas Scale in Office
As we continue to use this modified NEI scale in clinical trials, there’s also an opportunity to bring these new methods to the office – and doing so is easier than ever. PIs and doctors of all skill levels can quickly and easily learn new grading scales right
from their laptops, after web-based training programs became the norm during the COVID-19 pandemic.
We’ve made amazing progress on the evolution of corneal fluorescence staining. While we continue to learn about and adopt these new scales, it’s important that clinicians use them in clinical trials and office settings to demonstrate how well they truly work.
References:
Clinical Ophthalmology (PubMed 2023 Mar 7;17:757-767. doi: 10.2147/OPTH.S398843. eCollection 2023. “Validation of a Modified National Eye Institute Grading Scale for Corneal Fluorescein Staining” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10007867/